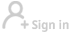EU regulator begins review of China's Sinovac coronavirus jab

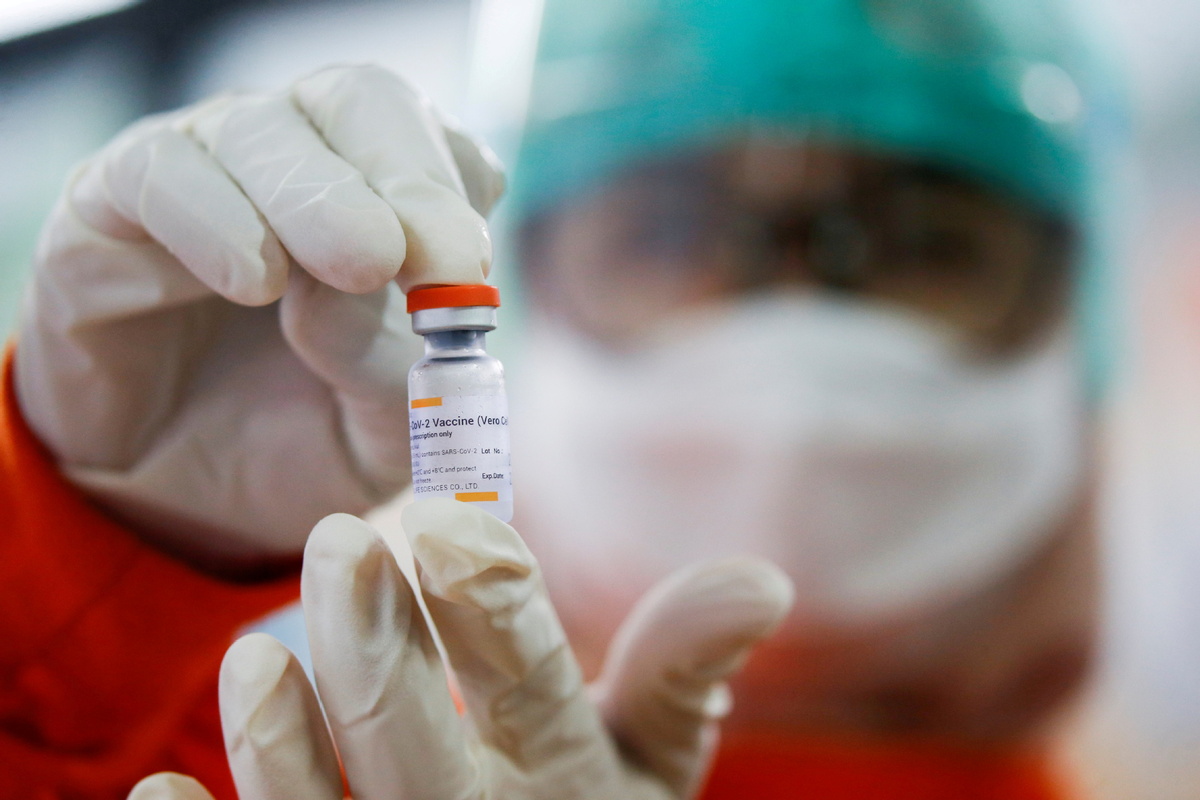
Europe's medicines regulator said on Tuesday it has started a real-time review of Sinovac's COVID-19 vaccine, based on preliminary results from animal and human trials that suggested the vaccine produces an immune response against the coronavirus.
Data on the vaccine, COVID-19 Vaccine (Vero Cell) Inactivated, will be assessed as they are made available to help speed up potential approvals, the European Medicines Agency (EMA) said.
This is the first Chinese vaccine the EMA is studying in real-time, and the fourth COVID-19 vaccine under such a review, including those from CureVac, Novavax Inc and Russia's Sputnik V.
Sinovac's vaccine has shown efficacy rates between 50 percent and 90 percent in different studies and is currently authorised for use in China, Indonesia, Brazil and Turkey.
The vaccine contains inactivated or dead versions of the SARS-CoV-2 virus to help the human body's immune system make antibodies.
In early April, Sinovac said its third production plant for its vaccine, branded CoronaVac in some regions, was ready, doubling its annual capacity to 2 billion doses. The firm said more than 200 million doses of Sinovac's vaccine have been delivered globally.
Rolling reviews are aimed at speeding up the approval process by allowing researchers to submit findings in real-time before final trial data is available.
Reuters